What Does the Ideal Gas Law Describe
A gas whose particles exhibit no attractive interactions whatsoever. R 83145 Jmol-1K-1.
Ideal Gas Law Study Guide Inspirit
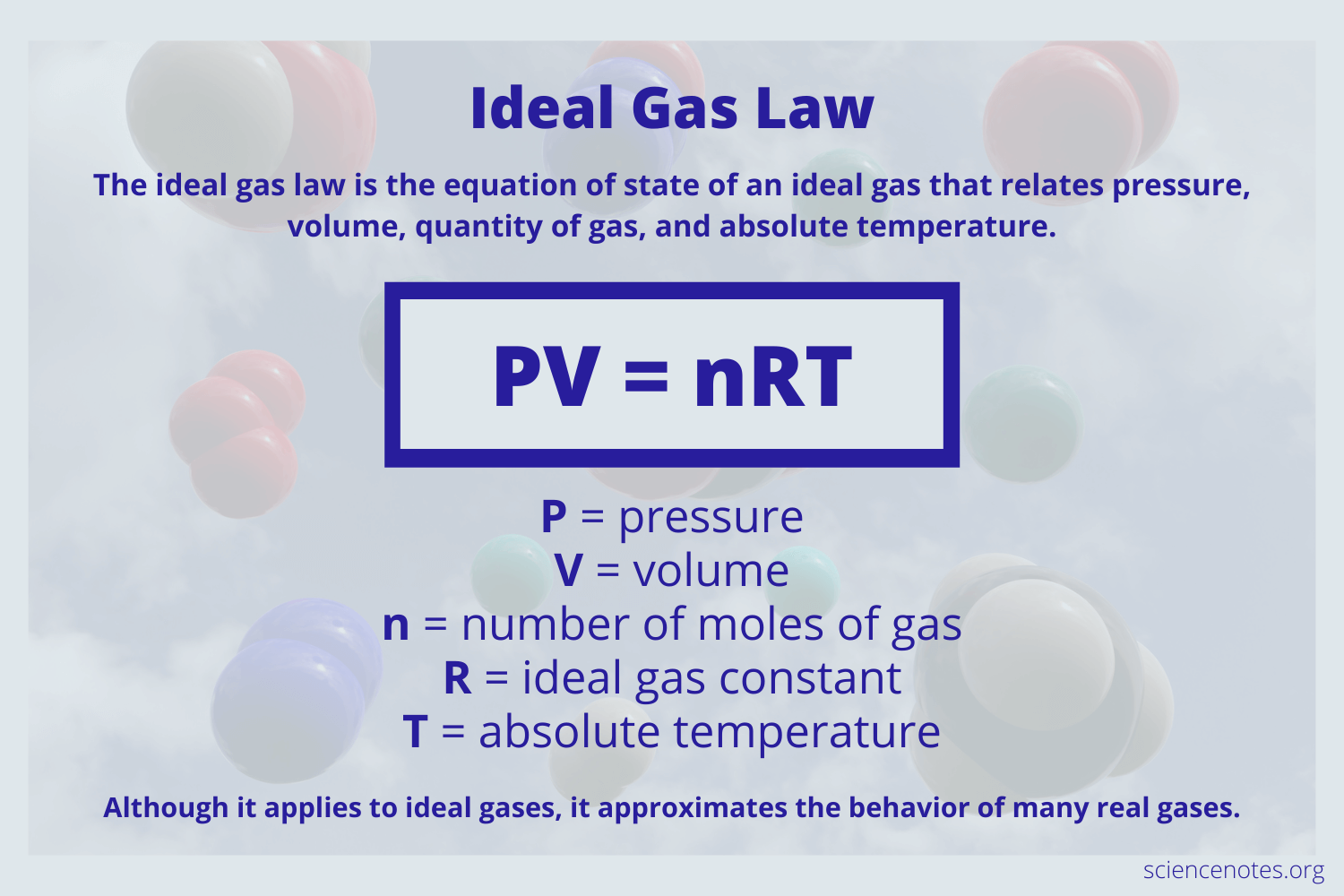
The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas.

. Describe the conditions in which the behavior of a real gas varies from that of an ideal gas. A physical law describing the relationship of the measurable properties of an ideal gas where P pressure V volume n number of moles R the gas constant T temperature in Kelvin. In application to chemical reactions this.
It is derived from a combination. All of the empirical gas relationships. Holiday weather albufeira April 18 2022.
The volume of 1 mol of an ideal gas at STP is 2241 L the standard molar volume. The Ideal Gas Law applies best to monoatomic gases at low pressure and high temperature. In the ideal gas equation both pressure and volume are directly proportional to temperature.
It is a good approximation of the behavior of many gases under many conditions although it has several limitations. The ideal gas law is also known as the general gas equation. Standard temperature and pressure STP is 0C and 1 atm.
The Ideal Gas Law states that in an ideal gas the relationship between pressure volume temperature and mass as PV nRT where P is pressure V is volume n is the number of moles a measure of. The Ideal Gas Law applies to ideal gases. 13-4 As we learned in the last lecture the gas laws of Boyle Charles Avogadro and Dalton merely explain the Kinetic-Molecular theory.
Intermolecular forces and molecular size are not considered by the Ideal Gas Law. The Ideal Gas Law is the relationship between pressure volume temperature and amount of gas. What does the ideal gas law describe.
The ideal gas law is a combined set of gas laws that is a thermodynamic equation that allows us to relate the temperature volume and number of molecules or moles present in a sample of a gas. The ideal gas law was discovered by physicist and engineer Benoît Paul Émile Clapeyron seen on the right in 1834. The ideal gas law can be written in terms of the number of molecules of gas.
The law was first stated by Émile Clapeyron in 1834. Ideal gas real gas gas constant. Buick commercial how do you stay so calm April 18 2022.
Chainsaw man volume 2 summary April 18 2022. An ideal gas contains molecules of a negligible size that have an average molar kinetic energy that depends only on temperature. The ideal gas law describes the behavior of an ideal gas a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases.
The law that the product of the pressure and the volume of one gram molecule of an ideal gas is equal to the product of the absolute temperature of the gas and the universal gas constant. What does the ideal gas law describe. At high temperatures and.
When a cloud forms the air parcel warms from the release. PV NkT where P is pressure V is volume T is temperature N is number of molecules and k is the Boltzmann constant k 138 10 23 JK. Nrage input plugin project 64 April 18 2022.
P₁V₁n₁T₁ P₂V₂n₂T₂ If one side of the equation is at STP then the data given for any reaction at STP will always give the value 008206 LatmmolK. While the law describes the behavior of a hypothetical gas it approximates the behavior of real gases in many situations. It is an equation of state of an ideal gas that relates pressure volume quantity of gas and temperature.
The ideal gas law also called the general gas equation is the equation of state of a hypothetical ideal gas. The Ideal Gas Law is derived from the combined gas law in the following way. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas.
State the ideal gas law and describe its variables.

Section 11 3 The Ideal Gas Law Ppt Download

The Ideal Gas Law Leave You In Awe The Bumbling Biochemist

Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry

Kinetic Theory Of An Ideal Gas Equation Assumption Concept Examples
No comments for "What Does the Ideal Gas Law Describe"
Post a Comment